Acid rain is over ten times more acidic than pure rainwater, causing damage to structures and buildings, but most importantly to vegetation (e.g. food crops). It also demineralises the soil, stunting the growth of plants. The acidity of the atmosphere is increasingly determined by carbon dioxide and organic acids such as formic acid. The second of these contribute to the formation of aerosol particles as a precursor of raindrops and therefore impact the growth of clouds and pH of rainwater. However, the chemical processes behind its formation were not well understood until now. A new study by an international team of researchers from the Université libre de Bruxelles (ULB) and the German research Centre Jülich (FZJ), with contributions from BIRA-IASB scientists, published in Nature on May 12th, finally sheds light on the formation mechanism of formic acid.
In the early 1980s, industrialised countries discovered that their forests were being ravaged by acid rain and that their trees were dying by the thousands. The cause was the emission of nitrogen oxides and sulphur oxides by human activity, which were reacting with water droplets in clouds to form sulphuric acid and nitric acid. Acid rain has a pH of about 4.2–4.8. This is over ten times lower than the pH of pure rainwater (5.5–5.7), which results from the natural carbon dioxide content of the atmosphere.
However, the chemical process that forms the bulk of the formic acid present in the atmosphere was unknown until present day. Dr. Bruno Franco (ULB) and Dr. Domenico Taraborrelli (FZJ) have now deciphered it:
chemical processes, impacts to air quality and climate, and measurement and analysis
tools used to analyse the effects of emissions changes. (Franco et al., 2021)
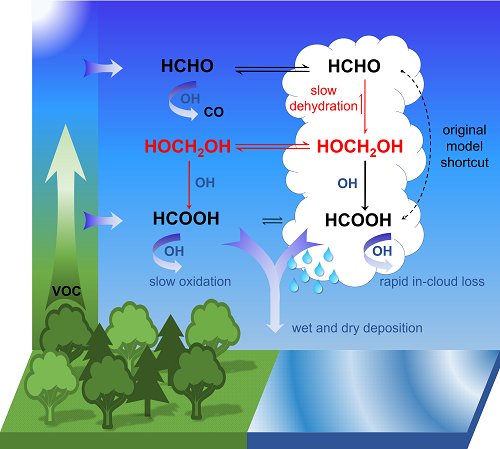
- Formaldehyde (HCHO) is formed naturally by photo-oxidation of volatile organic compounds (emitted by vegetation and certain industrial processes).
- Formaldehyde reacts in cloud droplets with water molecules to form methanediol (CH2(OH)2).
- The majority of this is outgassed and reacts with OH radicals, sometimes called the “detergent of the atmosphere”, in a photochemical process to form formic acid (HCOOH or CH2O2).
- A smaller portion also reacts with the liquid phase of the water droplets to form formic acid that is spread by rain.
“According to our calculations, the oxidation of methanediol in the gas phase produces up to four times as much formic acid as what is produced in other known chemical processes in the atmosphere,” says Domenico Taraborrelli. This amount reduces the pH of clouds and rainwater by up to 0.3, which highlights the contribution of organic carbon to the natural acidity in the atmosphere.
The scientists first tested their theory using MESSy, a global atmospheric chemistry model, and compared the results with the quantities of formic acid present in the atmosphere, calculated at the ULB thanks to the measurements of the IASI instrument (Infrared Atmospheric Sounding Interferometer) on board the Metop-A, -B and -C satellites. "These satellite measurements are very important because, as with other constituents of the atmosphere, they make it possible to assess the accuracy of models. And for the first time, the mechanism discovered makes it possible to match the models and the measurements of formic acid, which were largely underestimated until now," explains Bruno Franco. Subsequent experiments in the SAPHIR atmospheric simulation chamber in Jülich confirmed these results.
“We assume that the mechanism demonstrated is also active in aqueous aerosols and applies to other organic acids such as oxalic acid, which are not adequately accounted for in atmospheric chemistry models to date,” says Taraborrelli. One of the effects of this could be an improved understanding of the growth of aerosol particles and the development of clouds.
The Royal Belgian Institute for Space Aeronomy contributed to this study by providing measurements of organic compounds in the atmosphere: [1] global satellite observations of formaldehyde with the OMI spectrometer (De Smedt et al., 2018); [2] ground-based measurements of formic acid at Ile de la Réunion with an FTIR instrument (Vigouroux et al., ACP, 2012).
Publication
B. Franco, T. Blumenstock, C. Cho, L. Clarisse, C. Clerbaux, P-F. Coheur, M. de Mazière, I. de Smedt, H.-P. Dorn, T. Emmerichs, H. Fuchs, G. Gkatzelis, D. W. T. Griffith, S. Gromov, J. W. Hannigan, F. Hase, T. Hohaus, N. Jones, A. Kerkweg, A. Kiendler-Scharr, E. Lutsch, E. Mahieu, A. Novelli, I. Ortega, C. Paton-Walsh, M. Pommier, A. Pozzer, D. Reimer, S. Rosanka, R. Sander, M. Schneider, K. Strong, R. Tillmann, M. van Roozendael, L. Vereecken, C. Vigouroux, A. Wahner, D. Taraborrelli; Ubiquitous atmospheric production of organic acids mediated by cloud droplets, Nature, https://dx.doi.org/10.1038/s41586-021-03462-x, 2021.
Acknowledgments
Original press release by Institute of Energy and Climate Research – Troposphere (IEK-8).
Adaptations and translations were coordinated with the Université Libre de Bruxelles (ULB).
BIRA-IASB acknowledges national funding from BELSPO and ESA through the ProDEx project TRACE-S5P (TRACE-S5P project).
Multi-sensor HCHO developments have been funded by the EU FP7 QA4ECV project (grant no. 607405).
References
[1] De Smedt, I., Theys, N., Yu, H., Danckaert, T., Lerot, C., Compernolle, S., Van Roozendael, M., Richter, A., Hilboll, A., Peters, E., Pedergnana, M., Loyola, D., Beirle, S., Wagner, T., Eskes, H., van Geffen, J., Boersma, K. F., and Veefkind, P.: Algorithm theoretical baseline for formaldehyde retrievals from S5P TROPOMI and from the QA4ECV project, Atmos. Meas. Tech., 11, 2395–2426, https://doi.org/10.5194/amt-11-2395-2018, 2018.
[2] Vigouroux, C., Stavrakou, T., Whaley, C., Dils, B., Duflot, V., Hermans, C., Kumps, N., Metzger, J.-M., Scolas, F., Vanhaelewyn, G., Müller, J.-F., Jones, D. B. A., Li, Q., and De Mazière, M.: FTIR time-series of biomass burning products (HCN, C2H6, C2H2, CH3OH, and HCOOH) at Reunion Island (21° S, 55° E) and comparisons with model data, Atmos. Chem. Phys., 12, 10367–10385, https://doi.org/10.5194/acp-12-10367-2012, 2012.
Scientific Contact
- Dr. Bruno Franco, Spectroscopie, chimie quantique et télédétection atmosphérique (SQUARES), Université libre de Bruxelles (ULB)
Tel : +32 498 74 87 72 ; Email : bruno (dot) franco (at) ulb (dot) be
Press Contacts
- (ULB) Mathieu Léonard, Service Communication, Université libre de Bruxelles
Tel: +32 2 650 48 50; E-mail : mathieu (dot) leonard-salle (at) ulb (dot) be - (BIRA-IASB) Dr. Karolien Lefever, Head of the Communication and documentation department at BIRA-IASB
E-mail: Karolien (dot) Lefever (at) aeronomie (dot) be


